Behavior of Gases
What is kinetic Theory?!?!?!
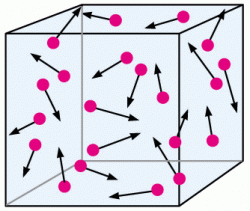
→Kinetic- Molecular Theory of gases is the study of behavior if gases term of particles in motion includ size, motion, and energy.
size
1) Gas partices are far apart, there are no significant repulsive force.
2) These particles are smaller than the distance between particles. The most of the volume of a gas is empty space.
Motion
1) Particles of gas are in random motion. They move in straight line until they collide with the walls.Collisions come out between as particles, elastic.
2) Elastic Collision is one that no kinetic energy is lost. The total kinetic energy doesn't change.
Energy
1)KE = 1/2 mv2. m is the mass of the particles, v is velocity.
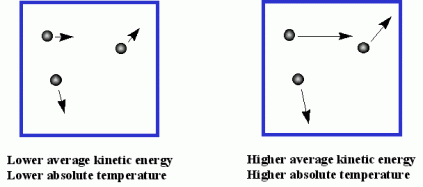
2)Kinetic energy and temperature are realted each other. At a given tmerpature, all the gases have the same average kinetic energy.
size
1) Gas partices are far apart, there are no significant repulsive force.
2) These particles are smaller than the distance between particles. The most of the volume of a gas is empty space.
Motion
1) Particles of gas are in random motion. They move in straight line until they collide with the walls.Collisions come out between as particles, elastic.
2) Elastic Collision is one that no kinetic energy is lost. The total kinetic energy doesn't change.
Energy
1)KE = 1/2 mv2. m is the mass of the particles, v is velocity.
2)Kinetic energy and temperature are realted each other. At a given tmerpature, all the gases have the same average kinetic energy.
Particles Energy is related to Temperature!
State of Matter ★
Solid

Molecules are held close to each other that they won't separate.
Liquid

Molecules will flow over one another but they stay toward in bottom of container.
Gas

Molecules are farther apart and move freely each other. When they collide each other, there would be no loss of energy.