Behavior of Gases
The Individual Gas Law_2
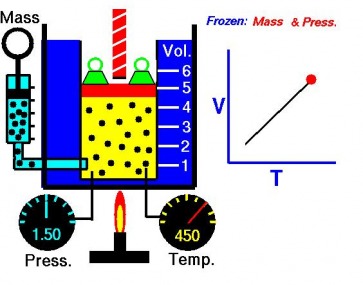
The Relationship of Temperature and Volume of Gas
Charles' Law describes the relationship of temperature and volume of a gas. The pressure does not change, a doubling in absolute temperautre of a gas causes a doubling of the volume of that gas. A drop of absolute temperatures see a proportional drop in volume. The volume of a gas increases by 1/273 of its volume at 0°C for every degree Celsius that the temperature rises.
The Formular of Charles' Law
* Temperature = Constant x Volume
or
* Volume = Constant x Temperature
or
* Volume/Temperature = Constant Substituting in variables, the formula is:
V/T=K ( equal to the constant)
* To solve for a change in volume or temperature using a proportion:
V2/T2 = V1/T1
or
V2/V1 = T2/T1
or
V1T2 = V2T1
So, if the distance increases, the volume increases. Therefore, volume and temperature are directly proportional.
The Formular of Charles' Law
* Temperature = Constant x Volume
or
* Volume = Constant x Temperature
or
* Volume/Temperature = Constant Substituting in variables, the formula is:
V/T=K ( equal to the constant)
* To solve for a change in volume or temperature using a proportion:
V2/T2 = V1/T1
or
V2/V1 = T2/T1
or
V1T2 = V2T1
So, if the distance increases, the volume increases. Therefore, volume and temperature are directly proportional.