Behavior of Gases
The Individual Gas Law_3
Joseph Louis Gay-Lussac

Gay-Lussac's Law is the third and final laws leading up to the gas law. The first law is Boyle's Law, which gives the relationship between volume and pressure, and the second is Charles' Law, which gives the relationship between volume and temperature. Gay-Lussac's Law has also been referred to as Charles' Law, but they are not the same.
Joseph Louis Gay-Lussac named Gay-Lussac's law in 1802.
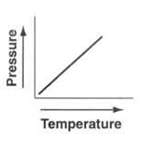
The pressure of a fixed mass and fixed volume of a gas goes directly proportional to the gas's temperature which is Kelvin.
The law can expressed in mathmatically = P/T=K
1. P1 / T1 = constant
2. P2 / T2 = constant( After the change in pressure and temperature)
3. P1 / T1 = P2 / T2 (Combined Two Equation)
P= Pressure T= The temperature K= Constant
